HCO barium hydroxide 7 8. It is a conjugate acid of a hydrogensulfate.

How To Write The Formula For Sulfurous Acid Youtube
According to Raman spectra of SO 2 solutions shows that the intensities of the signals are consistent with the equilibrium as follows.
. Write the compound name of the formula below IF5. It is a toxic corrosive and non-combustible compound. View the full answer.
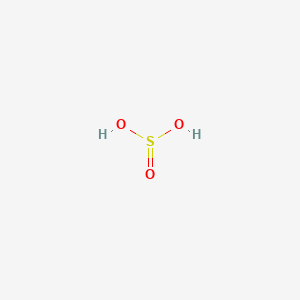
HBr aq Sulfurous acid. The chemical structure of the molecule is also given below where on sulfur atom is connected with one oxygen atom by double bond and two hydroxyl groups by single bonds only. Name the following acid.
The chemical formula of sulfurous acid is H 2 SO 3 and its molar mass is 8207 gmol. HCN aq Chloric acid. Its chemical structure is shown below.
The chemical formula of Sulfurous Acid is H 2 SO 3 while the formula for Sulfuric Acid is H 2 SO 4. Sulfurous acid is the chemical compound with the formula H2SO3Sulfuric or sulphuric acid H2SO. In this video well write the correct formula for Sulfuric acidTo write the formula for Sulfuric acid well use the Periodic Table a Common Ion Table and.
Name the following acid. SO 2 H 2 O HSO 3 H where K a 15410 2 and pK a 181. Corrosive to metals and tissue.
The chemical formula for sulfurous acid is H2SO3. It has a role as a catalyst. Sulfurous Acid Chemical Formula.
Sulfurous acid can be represented as In the structure of sulfurous acid the sulfur atom is in the centre with the three oxygen atoms bonded to it. H 2 SO 3. These two are composed of same chemical elements however in different ratios as you can see in their formulas.
Sulfuric acid spent appears as a black oily liquid. Its CAS number is 7782-99-2. This compound is hard to detect in solution but it is most likely found in a gaseous state.
8 rows Chemical Safety. The molecular formula shows that there are a total of two hydrogen atoms a single sulfur atom and three oxygen atoms. Sulfurous acid has the formula H 2 SO 3 and the molecular weight of 82075 gmol.
Sulfuric acid is a sulfur oxoacid that consists of two oxo and two hydroxy groups joined covalently to a central sulfur atom. The chemical formula of sulfurous acid is rm H_2 rm S rm O_3 It contains two atoms of hydrogen one atom of sulfur and three atoms of oxygen. Sulfurous Acid Health Hazards.
The Sulfuric acid is one of the quite important minerals having a wider range of industrial applications too. The chemical formula for the substance is written as H2SO3 with a molecular weight 8207 gmol. The chemical formula for the acid is given as H2SO4 with a molecular weight 98079 gmol approximately.
The eqH_2SO_3 eq compound name is called the sulfurous acid. There is no clear evidence that sulfurous acid exists in solution but the molecule has been detected in the gas phase. Write the formula for the following chemical compound.
The sulphur atoms are bounded with two oxygen atoms with double bonds and two hydroxyl group OH as shown below. 100 13 ratings H. Write the correct name or formula for the acids and bases below.
Magnesium hydroxide HCI 13. Sulfurous acid is a weak inorganic acid which is considered an aqueous solution of sulfur dioxide in water. This is the best answer based on feedback and ratings.
You may need your polyatomic ion chart. You may need your polyatomic ion chart. Laboratory Chemical Safety Summary LCSS Datasheet.
The chemical formula for sulfurous acid is. It consists of a sulfur atom having two single bonds with hydroxyl groups and one double.

How To Write The Formula For Sulfuic Acid Youtube

How To Balance H2so3 Naoh Na2so3 H2o Sulfurous Acid Sodium Hydroxide Youtube

0 Comments